Introduction
Arynes are reactive intermediates derived from substituted arenes
which participate in some aromatic nucleophilic substitutions and some
cycloaddition reactions. It is non isolable very reactive species with the
hexagonal planar ring structure with six pi delocalizing and 2 additional pi
electrons. The additional 2 pi electrons do not affect the aromatic character
of ring as it does not interfere with the Huckel number. Arynes are so highly
reactive that they exist as stable species only at very low temperatures, e.g.,
8 K for benzyne. Arynes may be derived from substituted benzenes, fused arenes
and pyridines.
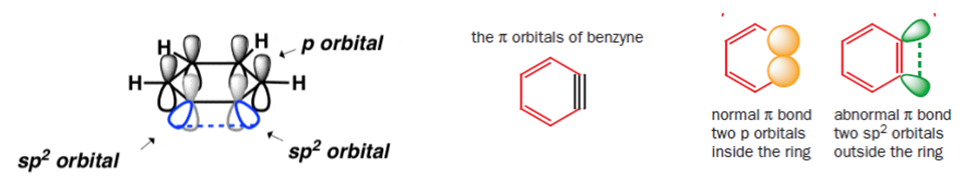
Structure of Benzyne
The sp2 orbitals involved in the ‘triple bond’ are at 90o to the p-orbitals of the aromatic pi-system Instead of an overlap between two 2p orbitals (as in an alkyne) the “triple bond” is formedthrough overlap of two adjacent sp2 orbitals in the plane of the ring (i.e., at right angles to, and completely independently of, the aromatic pi system poor overlap leads to a very weak triple bond, easily broken by a nucleophile.
Synthetic
Application of Arynes
Synthesis
of substitution Phenanthrene
Reactions of substituted phenyl acetylene with benzyne yields substituted phenanthrene.
Synthesis
of Aporphine Alkaloid
3,4-dimethyoxy acetylene reacts with benzyne, followed by H-migration to give the dimethoxyphenanthere. Similarly, 3-methoxybenzyne undergoes D-A reaction with aryl alkene to give aporphine alkaloids.
Synthesis
of Xanthene derivative
Aryl trifolone can be prepared by inserting aryne into R-SO2CF3
Synthesis
of Taxodione
Taxodione
was synthesized by [2+2] cycloaddition between an aryne and di-methoxyethylene
Synthesis
of Ellipticine
Antitumor alkaloid Ellipticine is synthesized by Diel’s alder addition to 3,4 pyridyne.
Synthesis of cyclobutene
Benzyne react with wide range of olefin to give [2+2] cycloaddition products.
Synthesis of aryl triflones
Aryl triflone can be prepared from arynes by inserting aryne into C-SO2F3, this reaction proceed via addition of C-SO2 F3 containing nucleophiles.
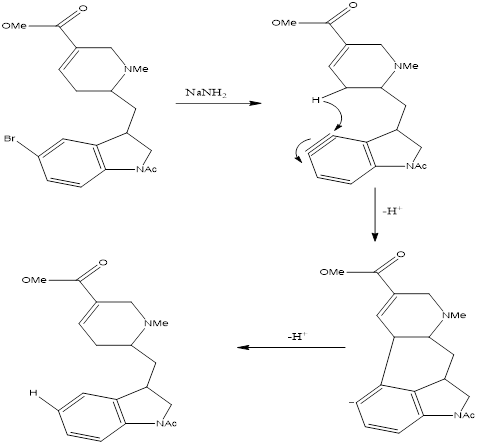
Synthesis of Lysergic acid N,N-diethylamide precursorReferences . Aten, C. F., and Greene, E. F. (1961). Combuetim & Flame 5,55.
Berry, R. S.,
Spokes, G. N., and Stiles, M. (1962). J. Am. Chem. SOC. 84, 3570
Bradley, J. N.,
and Kistiakowsky, G. B. (1961). J. Chem. Phye. 35,264.
Fields, E. K.,
& Meyerson, S. (1968). Mechanisms of formation and reactions of arynes at
high temperatures. Advances in Physical Organic Chemistry, 6, 1-61.











Comments
Post a Comment